Press Releases
A New Era in Biotechnology : What are Biomedicines?
Today, biomedicine is noted as a core engine for medical progress and plays an integral role in the healthcare industry.
According to Evaluate Pharma, a global pharmaceutical market research firm, the global biopharmaceutical market size is expected to surpass USD 300 billion (approx. KRW 331 trillion) this year. This is more than doubling from USD 151 billion (approx.. KRW 155 trillion) in 2012.
As we live in a new era of biotechnology, now more than ever, how we understand diseases and where we seek cures are becoming extremely important. In this article, we will go into detail about what are biomedicines, how they are developed and manufactured, and how they affect people’s lives.

Biomedicines are therapeutic drugs that are
produced in living organisms in the forms of antibodies, recombinant proteins,
and cell and gene therapies. Derived from living organisms, biomedicines have
lower toxicity and side effects, and are much higher in efficacy to target
specific chronic illnesses such as cancer, autoimmune disease, and Alzheimer’s
disease, etc.
The most common biomedicine is insulin, which is a hormone that controls the amount of glucose in your bloodstream and plays an important role in regulating the body's metabolic system. A rise in blood sugar levels would lead a typical person’s pancreas to release insulin. However in case of abnormal insulin secretion, a person’s blood sugar level may dangerously rise and cause diabetes, in which case insulin injection would be administered to manually control the blood sugar level.

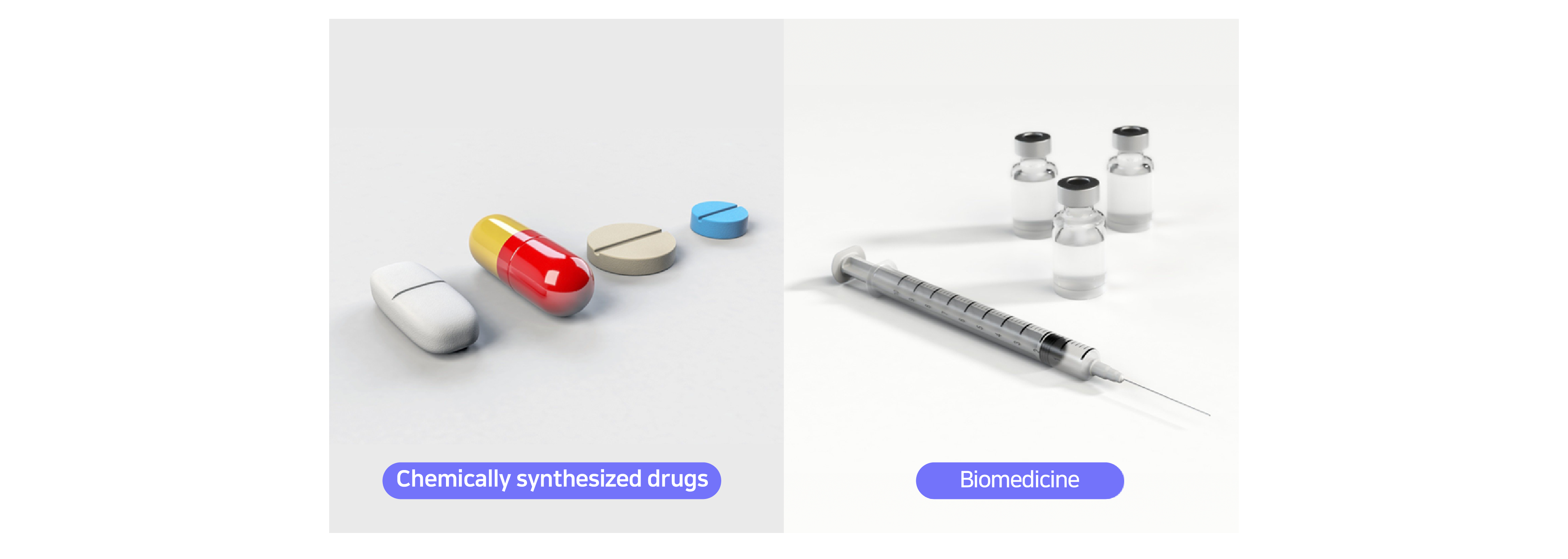
Biomedicines are different to chemically synthesized drugs. Chemically synthetized drugs are commonly known for medications you would take for headaches or indigestion and are generally administered orally. They are relatively smaller in size (molecular weight), simpler in terms of structure, and are less target specific with higher side effects.

The principle of immunization through vaccination was the initial mechanism that was employed for the first-ever biomedicine.
For many centuries, smallpox devastated mankind. In the 18th century, Edward Jenner, an English physician and scientist, took interest in the protective effects of cowpox. He had found that dairymaids were protected from smallpox naturally after having suffered from cowpox. Based on this, Jenner had concluded that cowpox not only protected people against smallpox, but also that it could be transmitted from one person to another as an intentional mechanism of protection. Through this research, he pioneered the concept of vaccines and demonstrated the first-ever scientific attempt to control an infectious disease through vaccination.

In the 19th century, Pasteur developed the overall principle of vaccines and further contributed to the foundation of immunology. He developed the vaccine for anthrax by attenuating the virus in rabbits and subsequently harvesting it from their spinal cords. In the 20th century, he continued to develop vaccines for plague, measles, rubella, varicella, and rotavirus.

Biomedicines can be largely classified as
vaccines, antibody therapy, cell and gene therapy, and cell therapy.
As previously mentioned, vaccines are a biomedicine that produce immunity to a specific disease by deliberately injecting a diluted pathogen into the body to create antibodies that will combat the disease.
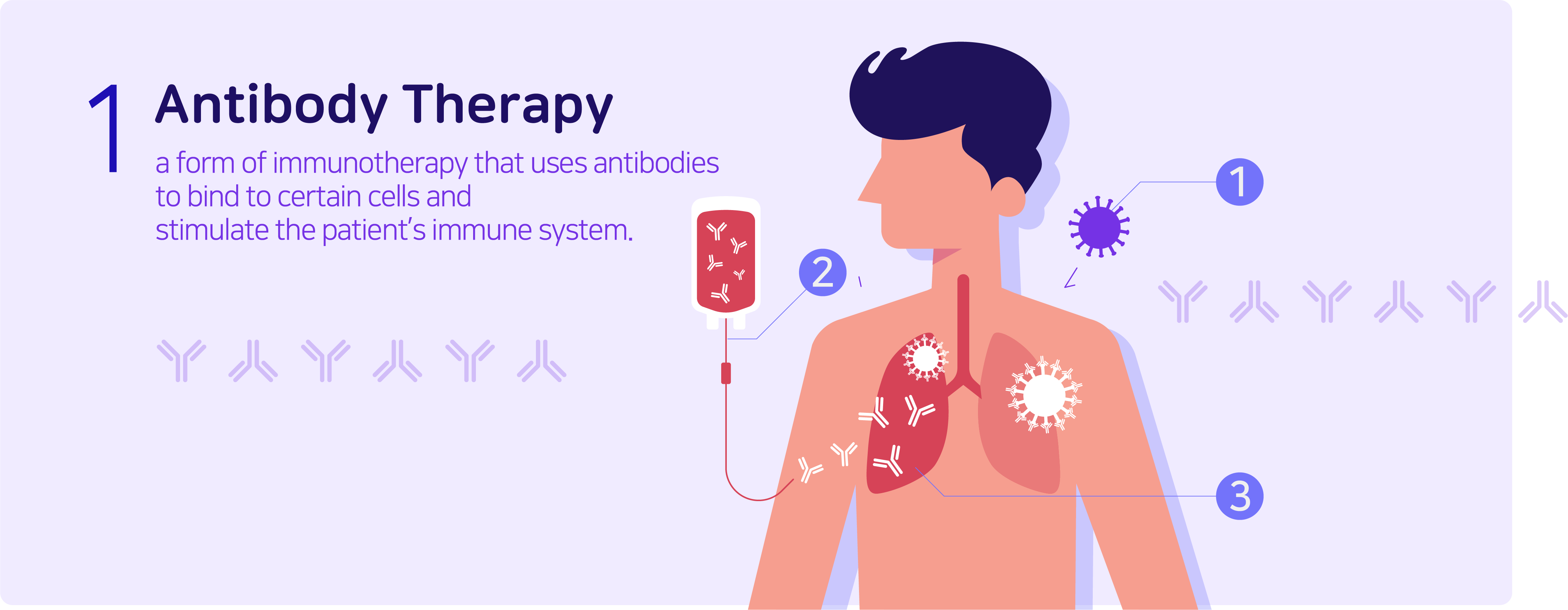
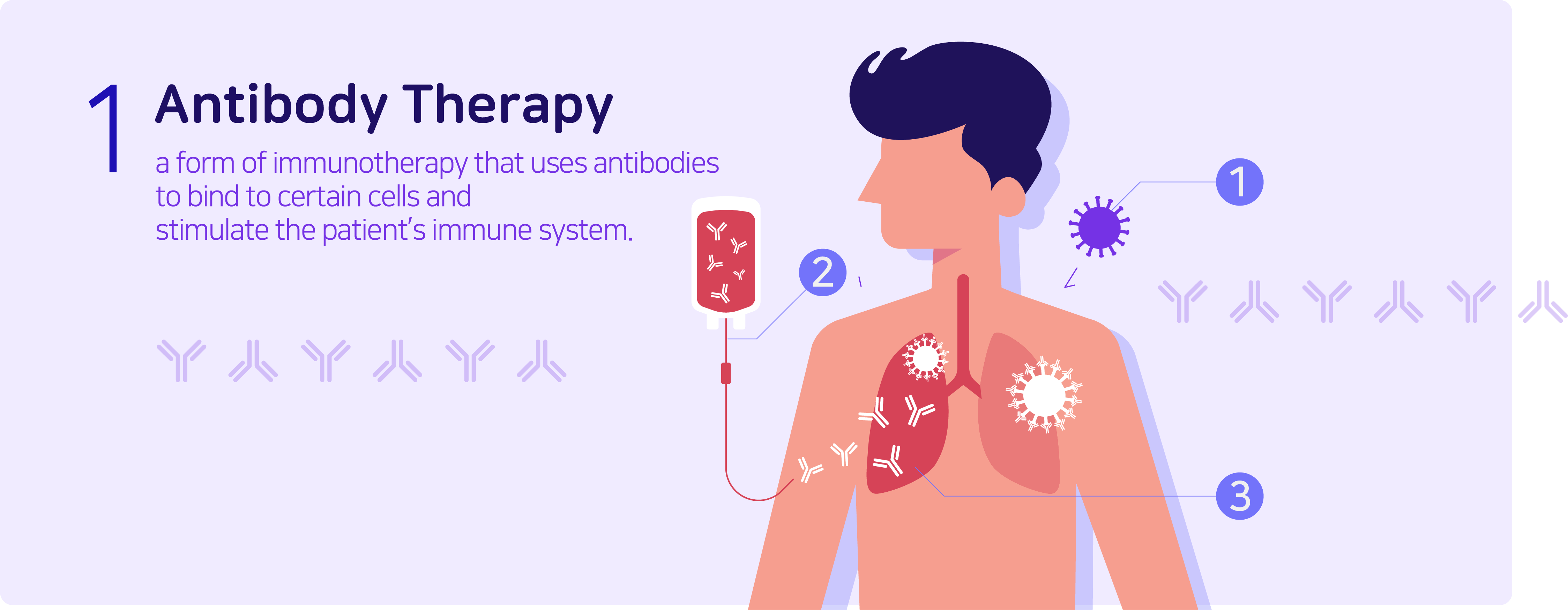
Antibody therapy is a form of immunotherapy that uses antibodies to monospecifically bind to certain cells or proteins to stimulate the patient’s immune system to attack the cells. Commonly known antibody therapies are Humira (autoimmune disease treatment), Remicade (rheumatoid arthritis treatment), Rituxan (blood cancer treatment), and Herceptin (breast cancer treatment). As a form of protein, antibodies can also be categorized as protein therapies, and are relatively stable in comparison to the latter.
* Antigen: A protein substance that triggers the immune system when it enters the body
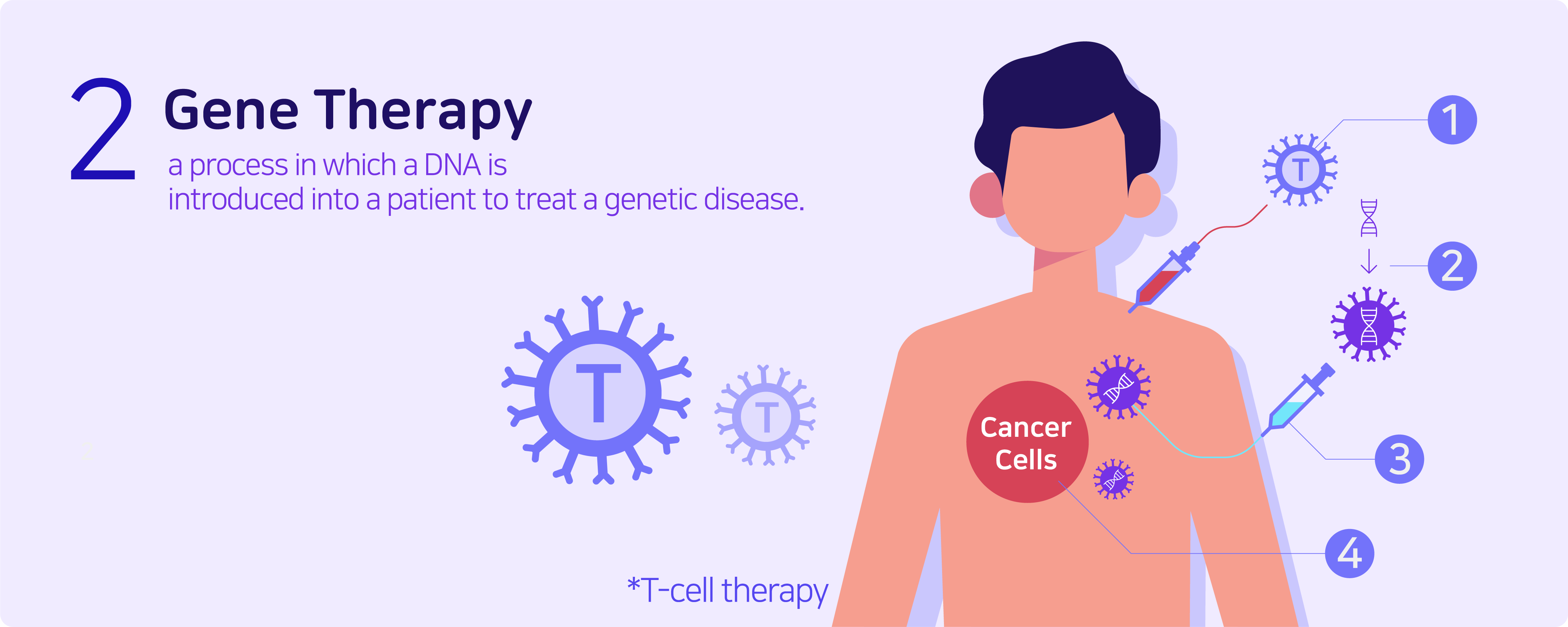
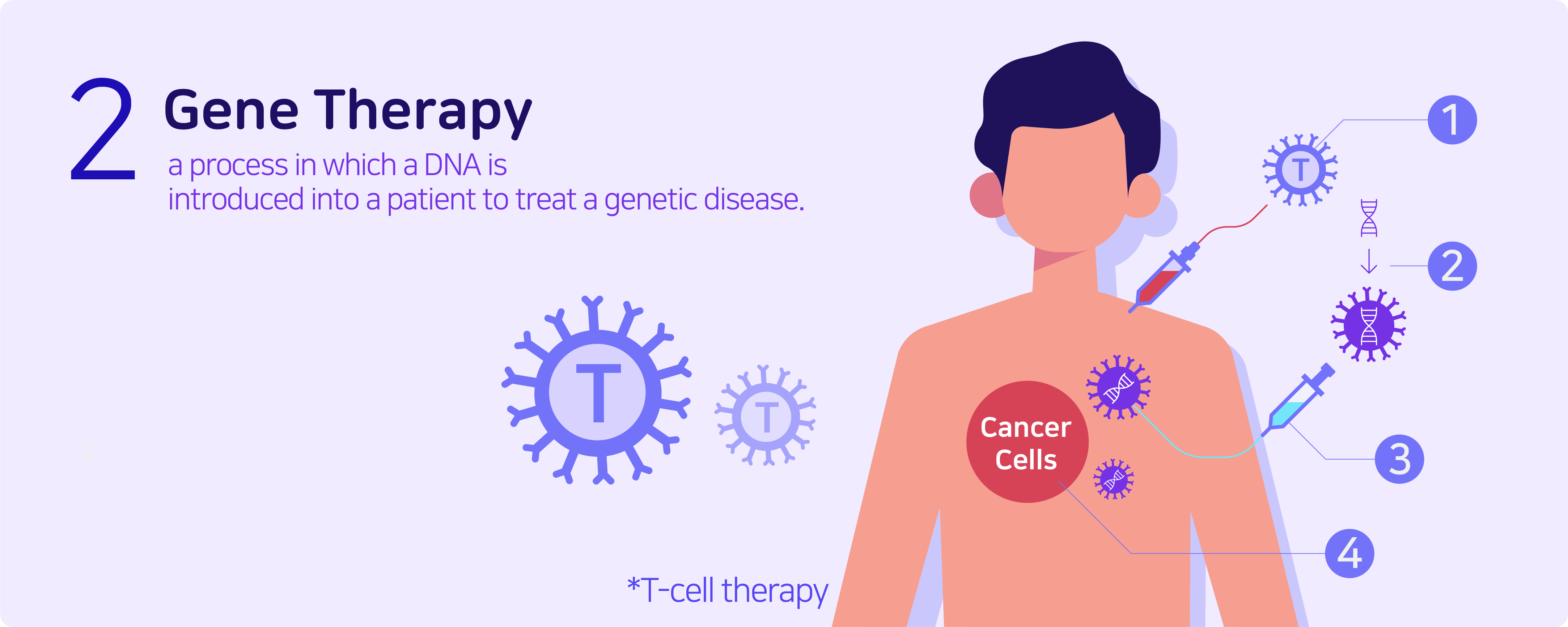
Genetic therapy is a process in which a DNA is introduced into a patient to treat a genetic disease. The new DNA usually contains a functioning gene to correct the effects of a disease-causing mutation. A gene that is inserted directly into a cell usually does not function. So instead, a carrier called a vector is genetically engineered to deliver the gene. Certain viruses are often used as vectors because they can deliver the new gene by infecting the cell. The viruses are usually modified to prevent any form of unwanted infection when used in people, however side effects can occur in certain cases.
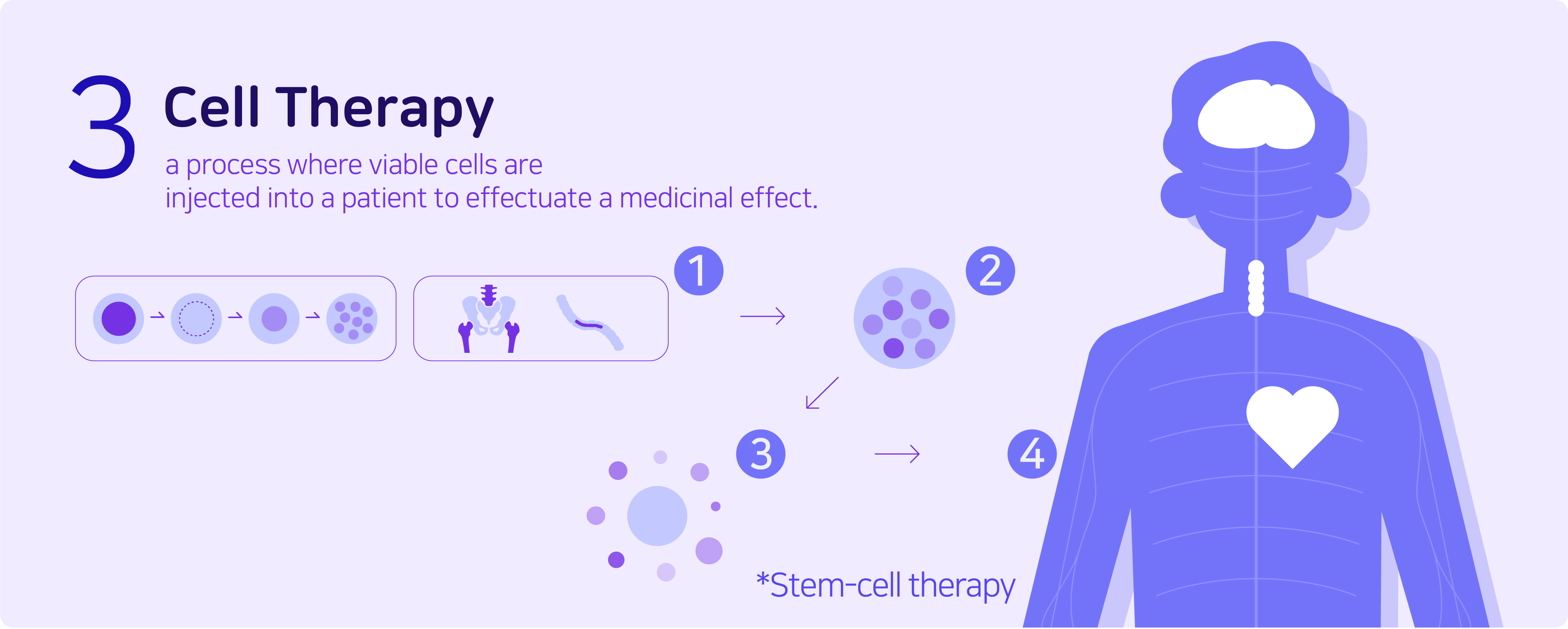
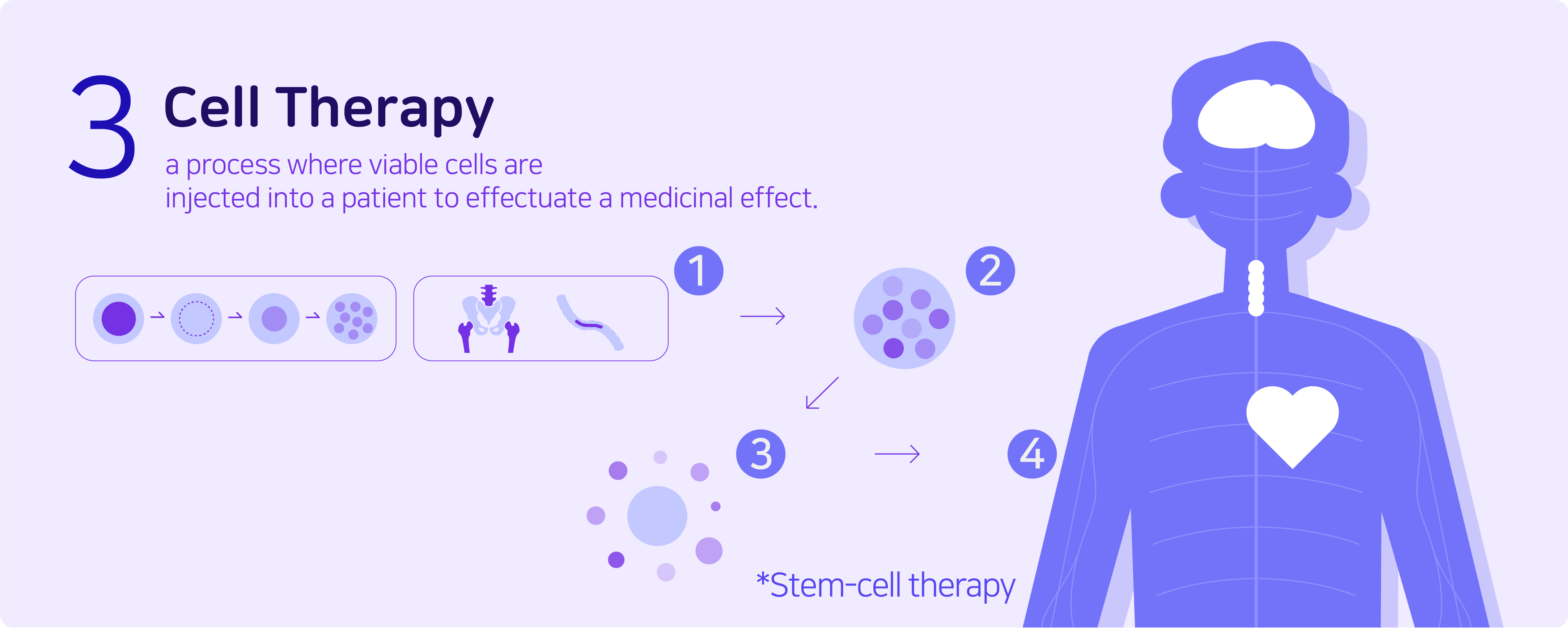
Cell therapy is a process where viable cells are injected into a patient in order to effectuate a medicinal effect. Stem-cell therapy is a commonly known example of a cell therapy that uses stem cells to treat or prevent a disease. This usually takes the form of a bone-marrow transplant, but the cells can also be derived from umbilical cord blood. The biggest advantage of this therapy is that there is an extremely low risk of side effects.

Samsung Biologics is a CDMO that collaborates
with some of the leading biotech companies worldwide to develop and manufacture
life-saving biomedicines. We have recently partnered with Eli Lilly and Company
and GSK to manufacture COVID-19 antibody therapies. We also have partnered with
other global biotech companies to develop and manufacture treatments for
chronic illnesses such as breast cancer, tumors, and cancer immunotherapy.
Samsung Biologics’ leading experts are working rigorously to expedite the delivery of high-quality products for our clients and patients to saves lives and make a positive impact on the healthcare landscape.
As we continue to experience disruptions worldwide, Samsung Biologics will continue with its ongoing efforts to provide end-to-end services to make biomedicines more accessible to all and bring a better future for humanity.
Today, biomedicine is noted as a core engine for medical progress and plays an integral role in the healthcare industry.
According to Evaluate Pharma, a global pharmaceutical market research firm, the global biopharmaceutical market size is expected to surpass USD 300 billion (approx. KRW 331 trillion) this year. This is more than doubling from USD 151 billion (approx.. KRW 155 trillion) in 2012.
As we live in a new era of biotechnology, now more than ever, how we understand diseases and where we seek cures are becoming extremely important. In this article, we will go into detail about what are biomedicines, how they are developed and manufactured, and how they affect people’s lives.

Biomedicines are therapeutic drugs that are produced in living organisms in the forms of antibodies, recombinant proteins, and cell and gene therapies. Derived from living organisms, biomedicines have lower toxicity and side effects, and are much higher in efficacy to target specific chronic illnesses such as cancer, autoimmune disease, and Alzheimer’s disease, etc.

The most common biomedicine is insulin, which is a hormone that controls the amount of glucose in your bloodstream and plays an important role in regulating the body's metabolic system. A rise in blood sugar levels would lead a typical person’s pancreas to release insulin. However in case of abnormal insulin secretion, a person’s blood sugar level may dangerously rise and cause diabetes, in which case insulin injection would be administered to manually control the blood sugar level.


Biomedicines are different to chemically synthesized drugs. Chemically synthetized drugs are commonly known for medications you would take for headaches or indigestion and are generally administered orally. They are relatively smaller in size (molecular weight), simpler in terms of structure, and are less target specific with higher side effects.

The principle of immunization through vaccination was the initial mechanism that was employed for the first-ever biomedicine.
For many centuries, smallpox devastated mankind. In the 18th century, Edward Jenner, an English physician and scientist, took interest in the protective effects of cowpox. He had found that dairymaids were protected from smallpox naturally after having suffered from cowpox. Based on this, Jenner had concluded that cowpox not only protected people against smallpox, but also that it could be transmitted from one person to another as an intentional mechanism of protection. Through this research, he pioneered the concept of vaccines and demonstrated the first-ever scientific attempt to control an infectious disease through vaccination.

In the 19th century, Pasteur developed the overall principle of vaccines and further contributed to the foundation of immunology. He developed the vaccine for anthrax by attenuating the virus in rabbits and subsequently harvesting it from their spinal cords. In the 20th century, he continued to develop vaccines for plague, measles, rubella, varicella, and rotavirus.

Biomedicines can be largely classified as vaccines, antibody therapy, cell and gene therapy, and cell therapy.
As previously mentioned, vaccines are a biomedicine that produce immunity to a specific disease by deliberately injecting a diluted pathogen into the body to create antibodies that will combat the disease.
Antibody therapy is a form of immunotherapy that uses antibodies to monospecifically bind to certain cells or proteins to stimulate the patient’s immune system to attack the cells. Commonly known antibody therapies are Humira (autoimmune disease treatment), Remicade (rheumatoid arthritis treatment), Rituxan (blood cancer treatment), and Herceptin (breast cancer treatment). As a form of protein, antibodies can also be categorized as protein therapies, and are relatively stable in comparison to the latter.
* Antigen: A protein substance that triggers the immune system when it enters the body
Genetic therapy is a process in which a DNA is introduced into a patient to treat a genetic disease. The new DNA usually contains a functioning gene to correct the effects of a disease-causing mutation. A gene that is inserted directly into a cell usually does not function. So instead, a carrier called a vector is genetically engineered to deliver the gene. Certain viruses are often used as vectors because they can deliver the new gene by infecting the cell. The viruses are usually modified to prevent any form of unwanted infection when used in people, however side effects can occur in certain cases.
Cell therapy is a process where viable cells are injected into a patient in order to effectuate a medicinal effect. Stem-cell therapy is a commonly known example of a cell therapy that uses stem cells to treat or prevent a disease. This usually takes the form of a bone-marrow transplant, but the cells can also be derived from umbilical cord blood. The biggest advantage of this therapy is that there is an extremely low risk of side effects.

Samsung Biologics is a CDMO that collaborates with some of the leading biotech companies worldwide to develop and manufacture life-saving biomedicines. We have recently partnered with Eli Lilly and Company and GSK to manufacture COVID-19 antibody therapies. We also have partnered with other global biotech companies to develop and manufacture treatments for chronic illnesses such as breast cancer, tumors, and cancer immunotherapy.
Samsung Biologics’ leading experts are working rigorously to expedite the delivery of high-quality products for our clients and patients to saves lives and make a positive impact on the healthcare landscape.
As we continue to experience disruptions worldwide, Samsung Biologics will continue with its ongoing efforts to provide end-to-end services to make biomedicines more accessible to all and bring a better future for humanity.