Quality Assurance
Samsung Biologics’ Quality Unit functions independently from Operations. The role of the Quality and Compliance department is to ensure that all CGMP processes comply with regulatory guidelines and requirements.
SYSTEM
- All CGMP processes comply with regulatory guidelines and requirements
-
With the quality systems, our quality and compliance department ensures that all equipment and systems are maintained throughout
CGMP production and our products are of high quality.
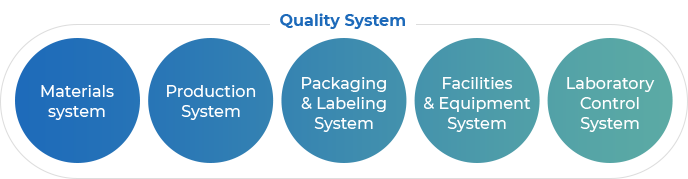
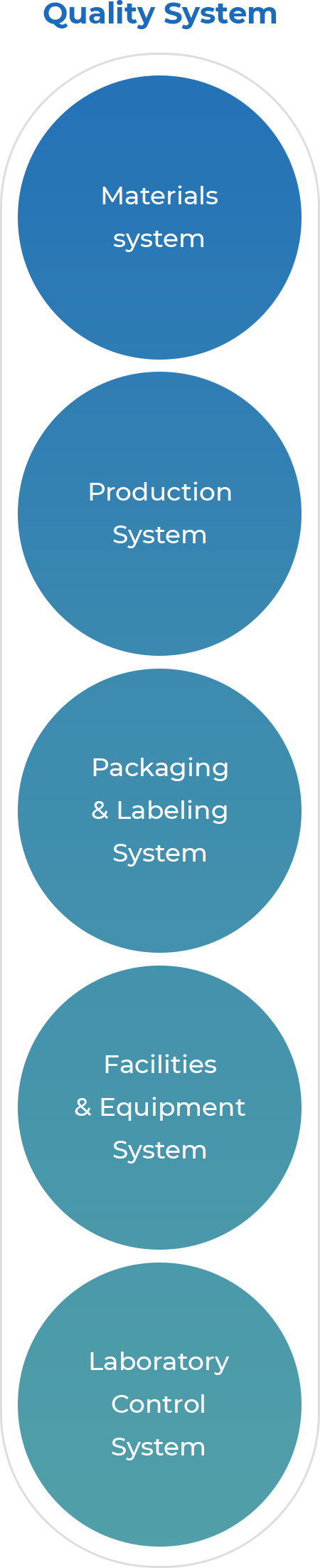 Quality System
Quality System- Materials system
- Production System
- Packaging & Labeling System
- Facilities & Equipment System
- Laboratory Control System
SYSTEM
- Implementation of a documentation management system
-
A documentation management system is in place for the controlled
generation, approval, and retention of all CGMP documents.
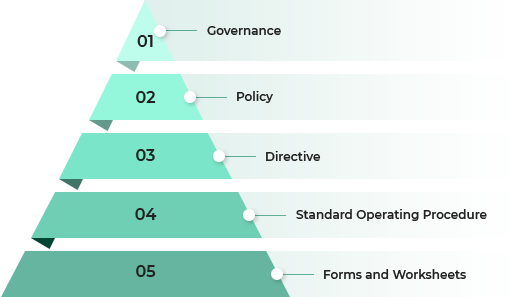
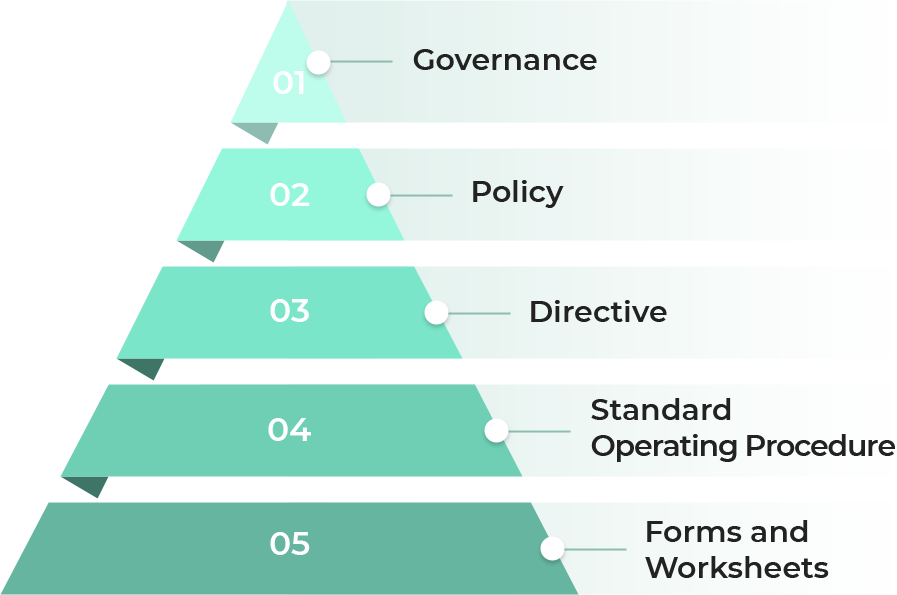
- 01 Governance
- 02 Policy
- 03 Directive
- 04 Standard Operating Procedure
- 05 Forms and Worksheets
Our Quality Systems ensure that all equipment and systems are thoroughly maintained throughout CGMP production and our products are of the highest quality. The Quality and Compliance department provides accurate oversight of raw materials, intermediates, products, packaging, labeling materials, and the proper maintenance of critical equipment.
Quality and Compliance reviews and approves all Specifications, Standard Operating Procedures (SOPs), Master Batch Records (MBRs), and investigations for any product or facility related deviations. We have an established training program, which guarantees that any personnel involved in the CGMP operations are qualified to do so. In addition, a documentation management system is in place for the controlled generation, approval, and retention of all CGMP documents. In order to operate effective quality systems, scheduled periodic internal audits and management reviews are conducted.
Samsung Biologics Quality & Compliance remains fully committed to the quality management that will produce continual improvements through the Corrective Action Preventive Action (CAPA) effectiveness checks and evaluation, Quality Risk Assessments (QRA), our supplier audit program, an in-process management review, and the self-inspection program. Through the activities of our Quality & Compliance department, Samsung Biologics maintains compliance with all international regulations for CGMP operations.