Press Releases
Accelerating success in biologics manufacturing | Tech transfer

To meet the needs of a growing and changing industry, a seamless bioprocess technology transfer is crucial for bringing drugs to market swiftly. However, this process presents challenges as issues can arise at every stage when transitioning manufacturing facilities and scales. Therefore, the ability of CDMOs to address risks and minimize time and costs becomes pivotal for successful commercialization.
This article explores how Samsung Biologics handles the complexities of tech transfers, leveraging its expertise and advanced technologies to assist clients in achieving their manufacturing goals.
Closing the technical gap from the early stage
The journey towards a successful tech transfer begins with an in-depth understanding of the project's unique requirements coupled with thorough planning and analyses. During the early stages, facility compatibility and operational gaps are meticulously assessed to ensure a smooth transition at every stage.
The facility fit assessment verifies key process parameters, including processing time and drug substance yield, and through the mass-balance approach we can identify critical potential risks. Operational differences and raw material risks are also identified through process and material gap analyses.
According to Hyejin Cho, Tech Transfer Senior Scientist, “High-risk items are addressed through mitigation plans, ensuring a systematic management of all gaps. Clarifying both initial clinical needs and potential commercialization requirements early in the process streamlines development, minimizes costs, and prevents delays down the line.”

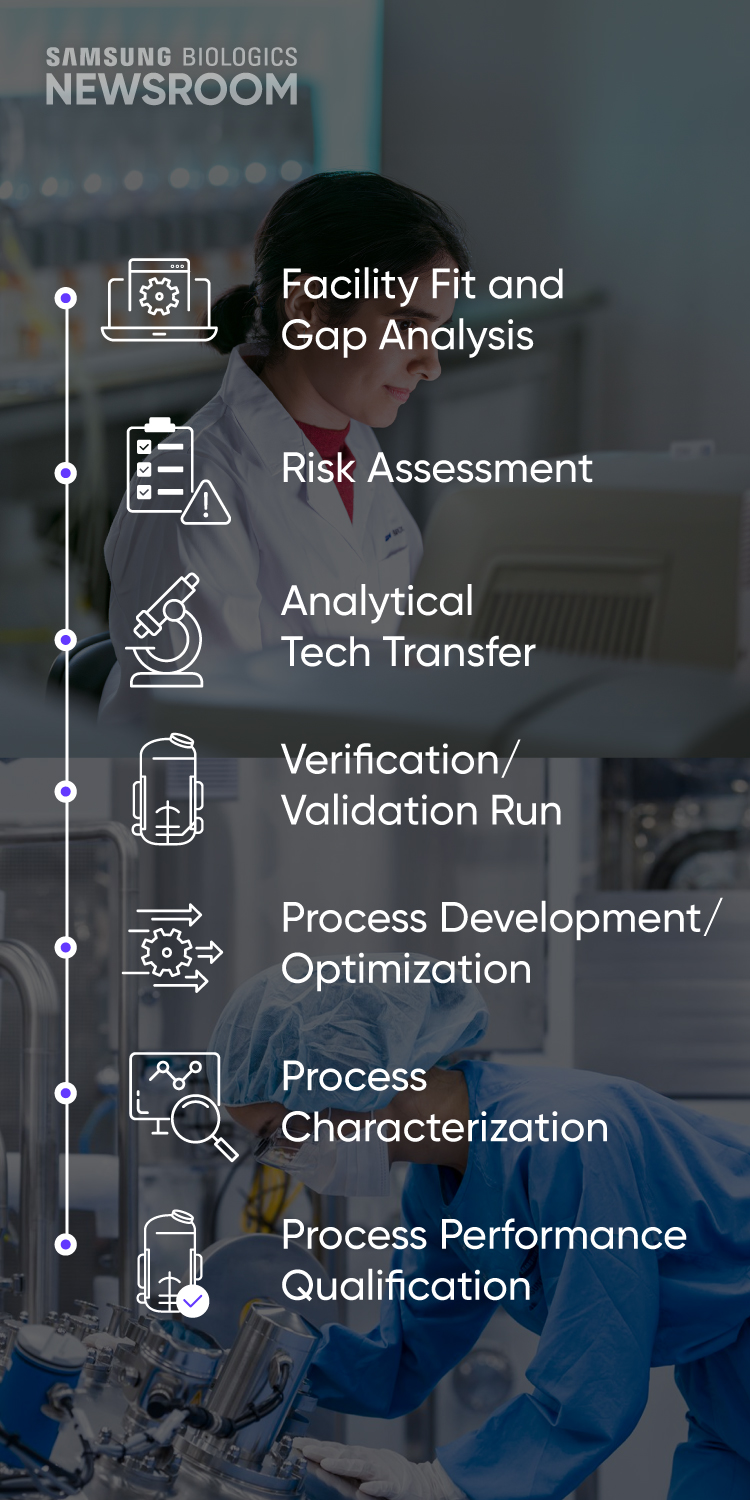
- Facility Fit and Gap Analysis
- Risk Assessment
- Analytical Tech Transfer
- Verification / Validation Run
- Process Development/ Optimization
- Process Characterization
- Process Performance Qualification
Strategic communication is paramount for success
Effective communication lies at the core of every successful tech transfer. Samsung Biologics’ dedicated team, comprising technical transfer scientists and project managers, as well as experts in quality assurance and regulatory compliance, maintains constant and transparent communication with clients. Regular meetings and thorough follow-ups ensure collaboration and information sharing, covering all technical aspects necessary for a seamless transfer.
"Closing the communication gap is crucial for mitigating potential risks throughout the process. By fostering open communication, we ensure our clients are well-informed at every stage. Collaborating closely, we address risks together, ensuring a seamless transfer," Cho highlighted.
To enhance the effectiveness, Samsung Biologics has embraced digitalization with the adoption of an Electronic Manufacturing Batch Record (eMBR) system. This system automatically captures data and monitors batch production in real-time, reducing errors and increasing accuracy in the manufacturing process. Additionally, Samsung Biologics provides a uniquely developed “BioProcess Library (BPL)” for clients, helping standardize process parameters and data into a unified document. Looking ahead, we aim to further streamline processes, prevent discrepancies, and enhance data integrity by integrating all related documents into the BPL system.
- 4F Future Space
- 3F 분석, 개발, 기술 이전
- 2F 세포독성약물 접합
- 1F 원자재 수급

A CDMO with integrated capabilities
By enhancing workflows with process optimization and digitalization of GMP documents, Samsung Biologics has achieved tech transfer timelines as short as three months, half the industry average of six months, allowing us to ensure on-time delivery.
“Our track record of success and collaborative efforts with global pharmaceutical companies underscore our dedication to innovation and continuous improvement. We proactively respond to market demand to deliver high-quality products on time despite a shifting regulatory landscape,” Cho added.
Through continued expansion into new modalities and manufacturing capacity, Samsung Biologics remains steadfast in its mission to achieve a better life for humanity. With unwavering commitment to excellence, Samsung Biologics stands as your trusted partner in swiftly and efficiently bringing life-changing drugs to market.
If you want to learn more about our tech transfer capabilities, read our whitepaper below.
- Innovative solutions for bioprocessing challenges: How MSAT drives efficient tech transfer
- Explore how we seamlessly execute tech transfers and scale-up processes, leveraging our robust MSAT expertise and advanced technologies, to ensure the success and quality of final products
Related Contents
Whitepapers Streamlining single-use assemblies in the bulk-fill step of downstream processing
Samsung BIO Insight Scaling Up Quality and Flexibility with Trusted Partnership

To meet the needs of a growing and changing industry, a seamless bioprocess technology transfer is crucial for bringing drugs to market swiftly. However, this process presents challenges as issues can arise at every stage when transitioning manufacturing facilities and scales. Therefore, the ability of CDMOs to address risks and minimize time and costs becomes pivotal for successful commercialization.
This article explores how Samsung Biologics handles the complexities of tech transfers, leveraging its expertise and advanced technologies to assist clients in achieving their manufacturing goals.
Closing the technical gap from the early stage
The journey towards a successful tech transfer begins with an in-depth understanding of the project's unique requirements coupled with thorough planning and analyses. During the early stages, facility compatibility and operational gaps are meticulously assessed to ensure a smooth transition at every stage.
The facility fit assessment verifies key process parameters, including processing time and drug substance yield, and through the mass-balance approach we can identify critical potential risks. Operational differences and raw material risks are also identified through process and material gap analyses.
According to Hyejin Cho, Tech Transfer Senior Scientist, “High-risk items are addressed through mitigation plans, ensuring a systematic management of all gaps. Clarifying both initial clinical needs and potential commercialization requirements early in the process streamlines development, minimizes costs, and prevents delays down the line.”
- Facility Fit and Gap Analysis
- Risk Assessment
- Analytical Tech Transfer
- Verification / Validation Run
- Process Development/ Optimization
- Process Characterization
- Process Performance Qualification
Strategic communication is paramount for success
Effective communication lies at the core of every successful tech transfer. Samsung Biologics’ dedicated team, comprising technical transfer scientists and project managers, as well as experts in quality assurance and regulatory compliance, maintains constant and transparent communication with clients. Regular meetings and thorough follow-ups ensure collaboration and information sharing, covering all technical aspects necessary for a seamless transfer.
"Closing the communication gap is crucial for mitigating potential risks throughout the process. By fostering open communication, we ensure our clients are well-informed at every stage. Collaborating closely, we address risks together, ensuring a seamless transfer," Cho highlighted.
To enhance the effectiveness, Samsung Biologics has embraced digitalization with the adoption of an Electronic Manufacturing Batch Record (eMBR) system. This system automatically captures data and monitors batch production in real-time, reducing errors and increasing accuracy in the manufacturing process. Additionally, Samsung Biologics provides a uniquely developed “BioProcess Library (BPL)” for clients, helping standardize process parameters and data into a unified document. Looking ahead, we aim to further streamline processes, prevent discrepancies, and enhance data integrity by integrating all related documents into the BPL system.
- 4F Future Space
- 3F 분석, 개발, 기술 이전
- 2F 세포독성약물 접합
- 1F 원자재 수급

A CDMO with integrated capabilities
By enhancing workflows with process optimization and digitalization of GMP documents, Samsung Biologics has achieved tech transfer timelines as short as three months, half the industry average of six months, allowing us to ensure on-time delivery.
“Our track record of success and collaborative efforts with global pharmaceutical companies underscore our dedication to innovation and continuous improvement. We proactively respond to market demand to deliver high-quality products on time despite a shifting regulatory landscape,” Cho added.
Through continued expansion into new modalities and manufacturing capacity, Samsung Biologics remains steadfast in its mission to achieve a better life for humanity. With unwavering commitment to excellence, Samsung Biologics stands as your trusted partner in swiftly and efficiently bringing life-changing drugs to market.
If you want to learn more about our tech transfer capabilities, read our whitepaper below.
- Innovative solutions for bioprocessing challenges: How MSAT drives efficient tech transfer
- Explore how we seamlessly execute tech transfers and scale-up processes, leveraging our robust MSAT expertise and advanced technologies, to ensure the success and quality of final products
Related Contents
Whitepapers Streamlining single-use assemblies in the bulk-fill step of downstream processing
Samsung BIO Insight Scaling Up Quality and Flexibility with Trusted Partnership
