Press Releases
Plant 5: Re-shaping biopharma operations
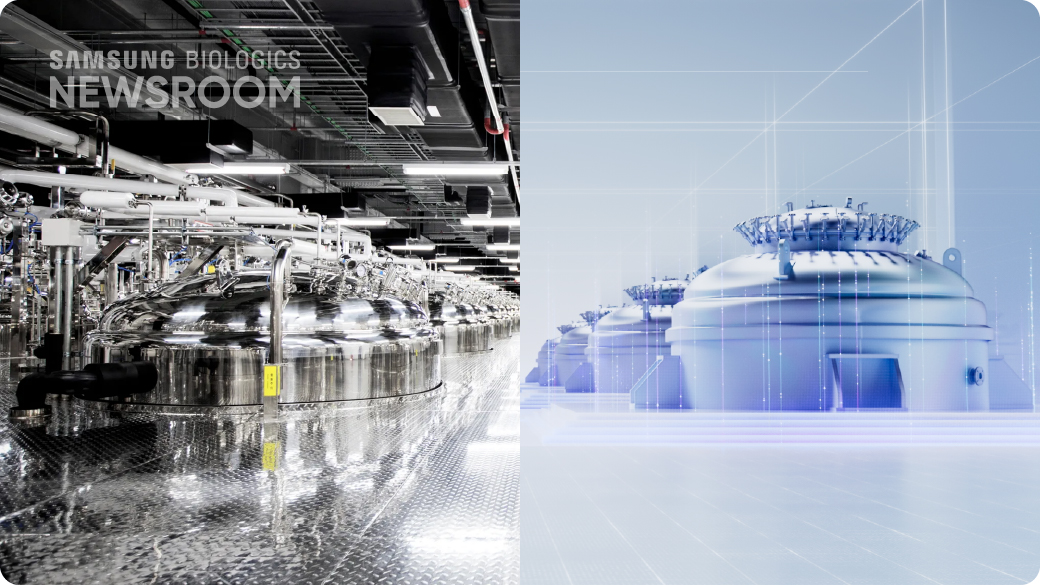
Maintaining its leadership in global biomanufacturing, Samsung Biologics continuously expands capacity while adding smart and innovative features to enhance operational excellence and technological advancements.
Having successfully met construction timelines, as committed, Plant 5, which opened this April, offers clients a new level of scalability and flexibility, adding a fresh 180 kL to bring the company’s current total manufacturing capacity to 784 kL.
Sustained momentum in building manufacturing capacity is complemented by digitalization and automation to equip clients with tools to embed quality into each manufacturing and development stage. With a proven track record and continuous commitment to setting the pace, Samsung Biologics focuses on addressing client needs to align with the global demand for reliable therapeutic supplies.
Designed to serve clients’ evolving needs
As new product launches often entail novel processes, legacy facilities may be constrained in adapting to the evolving needs of modern biomanufacturing. While Plant 5 follows a standardized design intended to serve as a future guide, it integrates innovative infrastructure to support both established and next-generation processes with agility and efficiency.
The core technologies, such as N-1 perfusion and Raman spectroscopy, along with advancements in digitalization, including computational fluid dynamics and digital twin technology, enable real-time data monitoring for clients. While these systems have already been standardized and implemented to form a strong foundation, Samsung Biologics goes further to pursue a facility design that prioritizes flexibility, allowing for the smooth incorporation of emerging technologies.

Equipping clients with operational excellence
One example of Samsung Biologics’ commitment to continuous improvement is logistics automation. Enhanced confidence in quality, which stems from the automation of once-manual procedures, contributes to robust manufacturing. Plant 4 started automating its storage and retrieval system in 2022; Plant 5 now applies end-to-end logistics automation across the entire site.
“If Bio Campus I was about automated storage, Bio Campus II would be about automated plants,” said Changgeun Lee, Associate Director of Plant 5 Logistics. Automation efforts, such as autonomous mobile robots (AMRs) in the central manufacturing support (MS) building on Bio Campus II, permeate the company.
The central MS building features a main spine bridge that acts as a linear highway, which will increase work efficiency by 50% upon campus completion. Separate routes are allocated for people and AMRs, which are programmed to transport supplies and samples to target locations, streamlining movement and ensuring employee safety.

Transparency in storage is made possible by real-time monitoring of supply and sample transportation. Multiple sensors in the storage system, for example, help prevent the fall or collision of materials and ensure supply quality while keeping the manufacturing process on schedule. Camera sensors and radio frequency identification labels for receiving goods allow for automated monitoring of supplies. Automated lifts, conveyor belts, stackers, and electronic pallet trucks simplify working environments, while a robot arm, once implemented, will automate sorting and disinfection.
Such process optimization helps employees stay engaged in relevant work while minimizing the potential for human error. And greater insights into how their molecules are progressing through each step of development and manufacturing empower clients to make well-informed decisions throughout the partnership.
We understand our clients’ critical need for consistency and minimal batch variation, supported by simplified, standardized, and flexible processes. Reliable execution of best practices in operations through the use of digitalized and automated systems benefits both clients and operators. The reduced potential for human error, in turn, lessens overall complexity throughout the facilities. Consistent quality is ensured, resulting in safe, quality medicines being delivered to patients worldwide.
Bio Campus II and beyond
Bio Campus II, where these advanced plant and storage systems take shape, will also house an Open Innovation Center. Samsung Biologics aligns with the needs of its current and future clients by fostering an ecosystem for biotech incubation and encouraging technological innovation. The Open Innovation Center will initially support biotechs by providing them with development services in the short term and manufacturing services in the long term.
“Having steadily evolved in offering services for diverse modalities, we continuously work to understand the flow of the market,” said Sangwon Seo, Vice President and Head of Engineering and Facility. “Just as the Amazonian rainforest is the lungs of the earth, Samsung Biologics and its global reach are like the lungs of the biopharma industry.”
Related Contents
Samsung BIO Insight Optimized operations for reliable biomanufacturing
Samsung BIO Insight Optimized facilities for flexible, agile manufacturing
About Us Bio Campus II

Maintaining its leadership in global biomanufacturing, Samsung Biologics continuously expands capacity while adding smart and innovative features to enhance operational excellence and technological advancements.
Having successfully met construction timelines, as committed, Plant 5, which opened this April, offers clients a new level of scalability and flexibility, adding a fresh 180 kL to bring the company’s current total manufacturing capacity to 784 kL.
Sustained momentum in building manufacturing capacity is complemented by digitalization and automation to equip clients with tools to embed quality into each manufacturing and development stage. With a proven track record and continuous commitment to setting the pace, Samsung Biologics focuses on addressing client needs to align with the global demand for reliable therapeutic supplies.
Designed to serve clients’ evolving needs
As new product launches often entail novel processes, legacy facilities may be constrained in adapting to the evolving needs of modern biomanufacturing. While Plant 5 follows a standardized design intended to serve as a future guide, it integrates innovative infrastructure to support both established and next-generation processes with agility and efficiency.
The core technologies, such as N-1 perfusion and Raman spectroscopy, along with advancements in digitalization, including computational fluid dynamics and digital twin technology, enable real-time data monitoring for clients. While these systems have already been standardized and implemented to form a strong foundation, Samsung Biologics goes further to pursue a facility design that prioritizes flexibility, allowing for the smooth incorporation of emerging technologies.

Equipping clients with operational excellence
One example of Samsung Biologics’ commitment to continuous improvement is logistics automation. Enhanced confidence in quality, which stems from the automation of once-manual procedures, contributes to robust manufacturing. Plant 4 started automating its storage and retrieval system in 2022; Plant 5 now applies end-to-end logistics automation across the entire site.
“If Bio Campus I was about automated storage, Bio Campus II would be about automated plants,” said Changgeun Lee, Associate Director of Plant 5 Logistics. Automation efforts, such as autonomous mobile robots (AMRs) in the central manufacturing support (MS) building on Bio Campus II, permeate the company.
The central MS building features a main spine bridge that acts as a linear highway, which will increase work efficiency by 50% upon campus completion. Separate routes are allocated for people and AMRs, which are programmed to transport supplies and samples to target locations, streamlining movement and ensuring employee safety.

Transparency in storage is made possible by real-time monitoring of supply and sample transportation. Multiple sensors in the storage system, for example, help prevent the fall or collision of materials and ensure supply quality while keeping the manufacturing process on schedule. Camera sensors and radio frequency identification labels for receiving goods allow for automated monitoring of supplies. Automated lifts, conveyor belts, stackers, and electronic pallet trucks simplify working environments, while a robot arm, once implemented, will automate sorting and disinfection.
Such process optimization helps employees stay engaged in relevant work while minimizing the potential for human error. And greater insights into how their molecules are progressing through each step of development and manufacturing empower clients to make well-informed decisions throughout the partnership.
We understand our clients’ critical need for consistency and minimal batch variation, supported by simplified, standardized, and flexible processes. Reliable execution of best practices in operations through the use of digitalized and automated systems benefits both clients and operators. The reduced potential for human error, in turn, lessens overall complexity throughout the facilities. Consistent quality is ensured, resulting in safe, quality medicines being delivered to patients worldwide.
Bio Campus II and beyond
Bio Campus II, where these advanced plant and storage systems take shape, will also house an Open Innovation Center. Samsung Biologics aligns with the needs of its current and future clients by fostering an ecosystem for biotech incubation and encouraging technological innovation. The Open Innovation Center will initially support biotechs by providing them with development services in the short term and manufacturing services in the long term.
“Having steadily evolved in offering services for diverse modalities, we continuously work to understand the flow of the market,” said Sangwon Seo, Vice President and Head of Engineering and Facility. “Just as the Amazonian rainforest is the lungs of the earth, Samsung Biologics and its global reach are like the lungs of the biopharma industry.”
Related Contents
Samsung BIO Insight Optimized operations for reliable biomanufacturing
Samsung BIO Insight Optimized facilities for flexible, agile manufacturing
About Us Bio Campus II